 Pin
Pin Image from Pixabay
At the core of everything you see—your coffee mug, your phone, your own hand—are atoms. And inside those atoms, the nucleus holds an insane amount of energy. We’re not talking lightning bolt-style visuals here, but more like the quiet, massive pressure of something incredibly dense waiting to break loose. Nuclear energy taps into that hidden force.
When you split a big, unstable atom like uranium-235, it breaks apart and throws off a chunk of energy and a few neutrons. Those neutrons hit other atoms, and boom—each one splits, releases energy, and throws more neutrons into the mix. This is a chain reaction, and it’s what happens inside a nuclear reactor.
The surprising part? It’s all controlled. Nothing like an explosion. Nuclear reactors slow down that reaction, capture the heat it gives off, and use it to make steam. That steam spins turbines, and those turbines generate electricity. It’s just water and heat—but at a crazy efficient level. The power of the atom turns into the light in your room.
Table of Contents
Fission vs. Fusion: The Two Powers Behind Nuclear Energy
 Pin
Pin Fission vs. Fusion / Image source: Climate and Hope
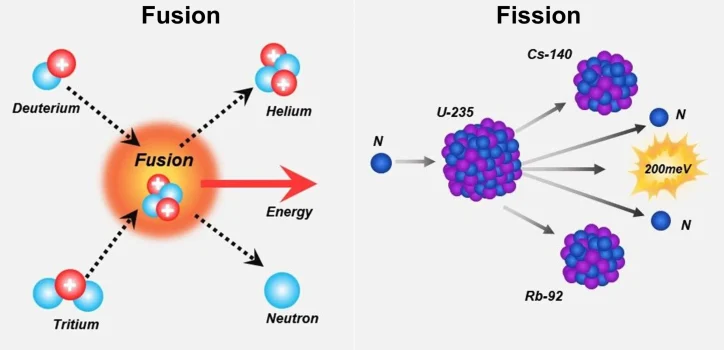
Nuclear energy might sound like a futuristic or even sci-fi concept, but at its core, it’s all about harnessing the energy in atoms. There are two main processes that make this possible: fission and fusion. Both are about releasing massive amounts of energy, but the way they do it is different.
Fission is the process most commonly used in nuclear reactors. It involves splitting a large, heavy atom, like uranium-235 or plutonium-239. When these atoms are hit by neutrons, they split into smaller atoms and release energy in the form of heat. This heat is used to boil water, which then produces steam to turn turbines and generate electricity. The key here is that the process creates a chain reaction—one that’s carefully controlled in reactors.
Fusion, on the other hand, is the process that powers the sun. It’s all about smashing light atoms, like hydrogen, together to form heavier ones, like helium. This releases a huge amount of energy. The catch? Fusion needs extreme conditions—far hotter than anything we’ve got on Earth, which makes it much harder to control for now.
Nuclear Energy in Action – How Power Plants Operate
When you think of nuclear power, it’s easy to picture something dangerous or out of control. But in reality, nuclear power plants are some of the most highly regulated and controlled facilities out there. The process is incredibly efficient, but it requires a fine balance to keep everything safe.
Inside a nuclear plant, fission is used to generate heat. This heat comes from the controlled splitting of atoms like uranium-235. The heat is then transferred to water, turning it into steam. This steam doesn’t just float around aimlessly; it’s directed into turbines. These turbines spin at incredible speeds, which then drives a generator to create electricity.
The most critical part of this process is maintaining the chain reaction. If the fission process speeds up too much, it can cause overheating, so reactors are designed with multiple safety mechanisms, including control rods that absorb neutrons and slow down the reaction when needed.
What makes nuclear power unique is how much energy comes from such a small amount of fuel. A single pellet of uranium-235 can produce as much energy as 3,000 tons of coal. That’s the kind of efficiency that keeps the world’s energy grid running.
The Pros and Cons of Nuclear Energy
Nuclear energy has always been a topic of intense debate. On one hand, it’s a powerhouse of efficiency and low-carbon power. On the other hand, the risks and challenges it brings are impossible to ignore. Let’s break down the pros and cons.
Pros
- Low Carbon Emissions: Nuclear power plants emit very little CO2 compared to fossil fuel plants. This makes them a huge asset in the fight against climate change.
- High Energy Density: As mentioned earlier, the energy output from nuclear fuel is enormous. A small amount of uranium can generate tons of electricity.
- Reliable Energy: Unlike solar or wind, nuclear power doesn’t depend on weather. It can produce energy 24/7, making it a steady, reliable source for grid systems.
Cons
- Radioactive Waste: The spent fuel from nuclear reactors remains dangerous for thousands of years. Finding safe, long-term storage solutions has been a persistent challenge.
- Risk of Accidents: While nuclear power plants are incredibly safe, accidents like Chernobyl and Fukushima remind us that the potential for disaster is real.
- High Costs: Building and maintaining nuclear plants is expensive, and the decommissioning process once they’re no longer in use adds to the cost.
The Future of Nuclear Energy – Is It the Key to Clean Power?
As we continue to search for sustainable energy solutions, nuclear energy is at a crossroads. While it’s already an important part of the energy mix in many countries, its future is uncertain. On one hand, it could be the key to reducing carbon emissions on a massive scale, but on the other, its challenges can’t be ignored.
The most exciting possibility for nuclear power is the development of small modular reactors (SMRs). These compact reactors are safer and cheaper to build than traditional large reactors, and they could be used in remote locations or even integrated with renewable energy sources. SMRs could make nuclear energy more flexible, safer, and more accessible than ever before.
Additionally, there’s the promise of nuclear fusion. Scientists are working on ways to harness fusion (the process that powers the sun), which could provide a virtually unlimited and clean energy source. If scientists can overcome the extreme heat and pressure required for fusion, it could revolutionize the energy sector.
While nuclear energy won’t be the only solution to our energy needs, it’s clear that its role in the future of clean, reliable power remains critical.
Does Nuclear Energy Create New Elements?
Nuclear reactions, whether through fission or fusion, do create new elements—that’s one of the core aspects of how nuclear energy works. When you split or combine atoms, the elements you start with can transform into entirely new ones. This is known as transmutation.
Take fission as an example: When uranium-235 undergoes fission, it splits into smaller elements like barium and krypton, both of which are completely different from uranium. These byproducts are not just waste—they’re new elements formed by the process of splitting the original atom.
In fusion, when hydrogen atoms combine to form helium, you’re again creating a new element. This process powers stars, including our sun, and holds great promise for future energy sources—though we still haven’t fully harnessed it here on Earth in a controlled way.
In both cases, nuclear energy doesn’t just provide power—it changes the very makeup of atoms, producing entirely new elements that weren’t there before.
Where Do We Get Radioactive Raw Materials on Earth?
Radioactive materials are naturally occurring and found all over the Earth, though they’re more concentrated in certain regions. These materials, like uranium, thorium, and radium, are essential for nuclear energy production. But where exactly do we get these raw materials?
The primary source of uranium and thorium is the Earth’s crust. Uranium, for example, is commonly found in rocks, particularly in granite and sedimentary rocks. One of the most abundant forms of uranium, Uranium-238, is widely distributed in nature, but it’s the rarer Uranium-235 that’s needed for nuclear reactions.
Plutonium, on the other hand, doesn’t naturally occur in large quantities on Earth. It’s mainly produced in reactors by irradiating uranium-238. This process makes plutonium-239, which is used in both reactors and nuclear weapons.
Countries with significant uranium deposits include Kazakhstan, Canada, and Australia, while India and Brazil are known for their thorium resources. These materials are extracted from mining operations that specialize in radioactive ores.
Does Radioactive Energy Get Absorbed in Any Way or Is It Infinite?
Radioactive energy is far from infinite, and it doesn’t simply keep on going without being absorbed or transformed. In fact, radioactive energy—whether from fission, fusion, or decay—follows strict laws of physics, and eventually, it’s absorbed, converted, or lost.
In nuclear power plants, the heat generated from fission reactions is absorbed by water, turning it into steam. This steam then drives turbines, which generate electricity. So, the energy is used and transferred in a controlled way. In this process, the energy from nuclear reactions isn’t “lost” but transformed into electricity, heat, and steam.
As for whether radioactive energy is infinite: no, it is not. The fuel used in nuclear reactors, like uranium or plutonium, eventually runs out. These materials are finite, and their energy potential is limited by the amount of radioactive material available.
Moreover, even though radiation can travel through materials (like when we see the effects of radioactivity on human tissues or other objects), it eventually loses its energy and decays over time. Nothing lasts forever in physics; it’s always being absorbed or converted into something else.
Is Nuclear Energy Too Risky for Humanity?
Nuclear energy certainly carries risks, and that’s part of the reason it’s such a controversial topic. On one hand, it provides a significant amount of clean energy with virtually zero carbon emissions. On the other hand, it also presents dangers that can have catastrophic consequences for both people and the environment.
The most well-known risks come from nuclear accidents. Accidents like Chernobyl and Fukushima have shown us the potential consequences of a nuclear meltdown. When a reactor fails, the release of radioactive materials can contaminate large areas, harm wildlife, and pose long-term health risks to humans, such as cancer from exposure to radiation.
There’s also the issue of radioactive waste, which remains hazardous for thousands of years. Finding safe, permanent storage solutions for this waste is an ongoing challenge. If not stored properly, radioactive waste could leak into the environment.
That said, modern reactor designs have made significant strides in safety, and many experts argue that the risks are manageable. With proper safeguards, regulations, and technology, nuclear energy can be a safe and reliable power source. But the inherent risks still make it a controversial choice.
How Carbon Dating Works – Unveiling the Past with Nuclear Science
 Pin
Pin Carbon Dating / Image source: sciencenotes.org
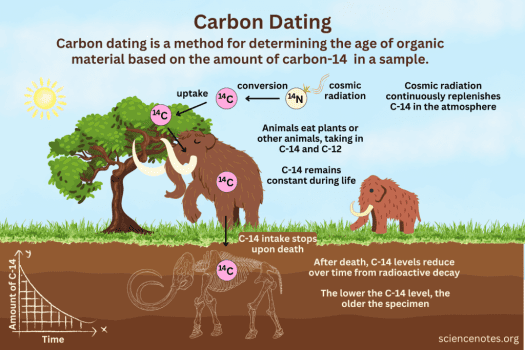
Carbon dating, or radiocarbon dating, is a powerful tool used by scientists to determine the age of ancient artifacts, fossils, and remains. The principle behind carbon dating is based on the fact that carbon-14, a radioactive isotope of carbon, is absorbed by living organisms during their lifetime. When an organism dies, it stops absorbing carbon, and the carbon-14 inside begins to decay at a predictable rate.
Carbon-14 decays into a stable form, nitrogen-14, over a known half-life of about 5,730 years. By measuring the amount of carbon-14 remaining in a sample and comparing it to the original amount in the living organism, scientists can estimate the time that has passed since the organism’s death.
This process works because carbon-14 is constantly replenished in the atmosphere through interactions with cosmic rays, ensuring that living organisms contain a consistent ratio of carbon-14 to carbon-12. However, carbon dating is only effective for objects up to about 50,000 years old. For older items, other methods like potassium-argon dating are used.
What Is Half-Life? Understanding the Clock of Radioactive Decay
Half-life is a term you’ll often hear when talking about radioactive materials and how long it takes for them to lose their energy or transform into another element. Simply put, a half-life is the time it takes for half of the atoms in a sample of a radioactive substance to decay. After one half-life, half of the atoms will have decayed, and the other half will still remain radioactive. After two half-lives, only a quarter of the original atoms remain.
This process is random at the level of individual atoms, meaning it’s impossible to predict exactly when a single atom will decay. However, on a large scale, half-lives help scientists calculate how much of a substance remains at any given time.
The length of a half-life varies widely depending on the substance. For example:
- Carbon-14 (used in carbon dating) has a half-life of around 5,730 years.
- Uranium-238 (used in nuclear reactors) has a half-life of over 4.5 billion years.
Understanding half-life helps in fields ranging from archaeology to nuclear energy to medicine, as it plays a crucial role in predicting how long radioactive materials remain hazardous.
FAQs
Nuclear energy is the energy stored in the nucleus (core) of atoms. This energy is released through two main processes: fission (splitting large atoms like uranium) and fusion (combining smaller atoms like hydrogen). In power plants, fission is used to generate heat, which turns water into steam, driving turbines to produce electricity.
While nuclear energy is generally safe, it does carry risks, such as potential nuclear accidents (e.g., Chernobyl or Fukushima). The primary dangers involve the release of radioactive materials, which can harm both the environment and human health. Additionally, the disposal of radioactive waste is a significant challenge due to its long-lasting hazard.
No, nuclear energy isn’t considered a renewable energy source because it relies on finite materials like uranium and plutonium, which will eventually be depleted. However, nuclear power is highly efficient compared to other energy sources and produces low-carbon emissions, making it a cleaner alternative to fossil fuels in the long term.
The half-life of a radioactive material is the time it takes for half of the atoms in a sample to decay. For example, carbon-14 (used in dating) has a half-life of about 5,730 years, while uranium-238 has a half-life of 4.5 billion years. The half-life depends on the specific material and its rate of decay.
Nuclear fusion is the process where two light atoms, like hydrogen, combine to form a heavier atom, such as helium, releasing a tremendous amount of energy. This process powers the sun. While fusion has the potential to provide virtually unlimited, clean energy, scientists are still working to make it feasible for use on Earth due to the extreme conditions required.






























